The Fluoride Exposed logo tells you a lot about who we are.
It shows what total science geeks we are!
chemistry!
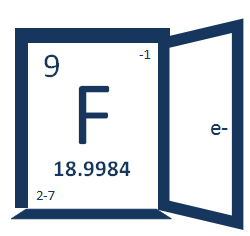
What is fluoride? It's not just a cavity fighter in fluoride toothpaste and fluoridated water. It's also an ion of an element (fluorine) on the periodic table. And the periodic table is one of the most fundamental science topics we build modern science on.
So we think it was just terribly clever to put the periodic table entry for fluoride inside a door for our logo. We also did the design ourselves! In a super geeky computer program called Gimp, too. Heh, we don't blame you if you think we should have hired a professional graphic designer, but don't let that distract you from the best parts of this logo ... the chemistry knowledge right there at the top of every page!
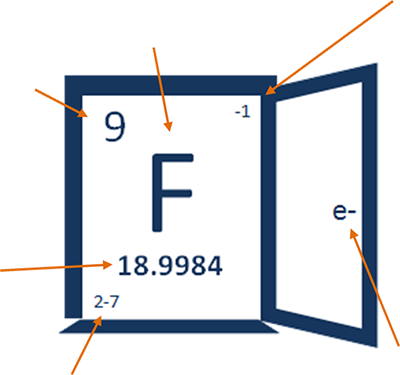
The number 9 is up in the left corner because fluoride is the 9th element on the periodic table. Oh, that's also how many protons are in the nucleus and how many electrons are in the cloud of a fluorine atom.
18.9984 – that's how many grams that 602,000,000,000,000,000,000,000 fluoride atoms weigh, a.k.a. atomic mass.
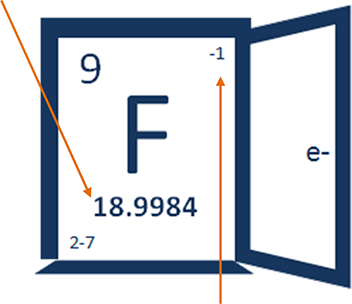
-1 tells you about the most common ion of fluorine in nature. In other words ... -1 tells you about fluoride!
Fluorine isn't really happy with only 9 electrons. It wants 10 electrons. We don't know about you, but if we only had 9 of something, we sure would want another to make it 10. Change due is 9 cents? Where is that take a penny/leave a penny jar, so we can get a nice dime back instead?
So fluorine wants an extra electron to make it 10. And when it gets that extra electron, it becomes fluoride. Fluoride is the ion of fluorine, and it has a charge of -1.
And because we are super dorks, we gave this extra electron that turns fluorine into fluoride a special place on our fluoride-door logo – we made it the doorknob!
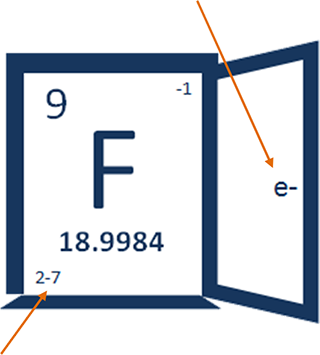
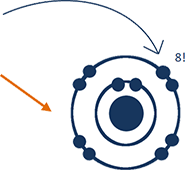
2-7 tells you more about the electrons in a fluorine atom and where they are.
The official term is electron configuration.
Two of fluorine's 9 electrons
are in the first shell,
and seven
are in the
second shell.
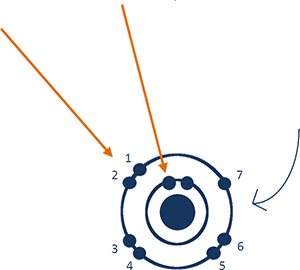
You'll see these bad boys around Fluoride Exposed, too, so here's a little bit about these graphics of the fluorine and fluoride atoms ....
and you get fluoride ...